Vitroids™ & Lenticules® Discs by Merck
Certified Reference Microorganisms (CRM)
-
REQUEST QUOTATION
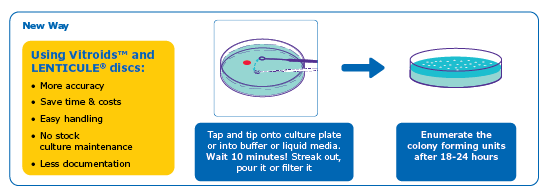
Vitroids™ and Lenticules® are discs containing accurately measured, viable microorganisms in easy-to-use formats. Just rehydrate and use, no need to prepare or maintain stock cultures.
Ideal for pharmaceutical, food & beverage, cosmetics, and clinical labs, they streamline microbiological quality control with reliable performance, traceability, and compliance.
- Certified Microbial Load: Each disc contains a known quantity of CFUs (Colony Forming Units), verified under ISO 17025 and ISO 17034 standards, with a Certificate of Analysis (CoA) detailing organism type, CFU range, uncertainty, and source strain.
- Traceable Strains: Derived from internationally recognized culture collections (e.g., NCTC, NCPF, CECT), ensuring genetic authenticity and regulatory compliance.
- Minimal Handling, Maximum Accuracy: Delivered in an easy-to-use format, reducing preparation time and the risk of contamination.
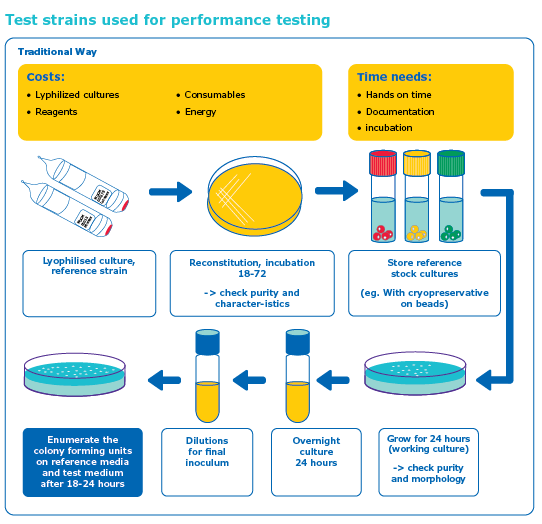
- Long Shelf-Life & Stability: Products are lyophilized for extended shelf life without compromising viability.
- Batch-to-Batch Consistency: Manufactured under strict quality controls to ensure homogeneity and repeatability.
vs
1. Method Validation / Verification
- Certified reference microorganisms used to establish or verify microbiological methods.
- Provide a known, traceable microbial load to ensure accuracy, precision, and regulatory compliance.
2. Routine Quality Control (QC)
- Ready-to-use reference discs for daily QC of media, reagents, and instruments.
- Eliminate the need for live culture maintenance while improving consistency and traceability.
3. Growth Promotion Testing (GPT)
- Provide standardized inoculum to confirm media support expected microbial growth.
- Ensure reliability and compliance with USP <61>/<62> and ISO 11133 requirements.
4. Training & Competency / Proficiency Testing
- Safe, stable materials for staff training, analyst competency, and proficiency testing.
- Support consistent technique and confidence without handling live cultures.

